Abstract
Background: The CD22-directed antibody-drug conjugate inotuzumab ozogamicin (InO) is FDA-approved for adults with relapsed/refractory (R/R) CD22+ B-ALL and highly active in children with multiply R/R CD22+ B-ALL. Notable toxicities include cytopenias and hepatic toxicity, including sinusoidal obstruction syndrome (SOS) occurring primarily after subsequent hematopoietic stem cell transplantation (HSCT). The incorporation of InO into frontline ALL therapy for patients (pts) with CD22+ high-risk B-ALL who are not anticipated to require HSCT in first remission may optimize use of this effective agent without incurring significant SOS risk by minimizing relapse and therefore potential need for future HSCT.
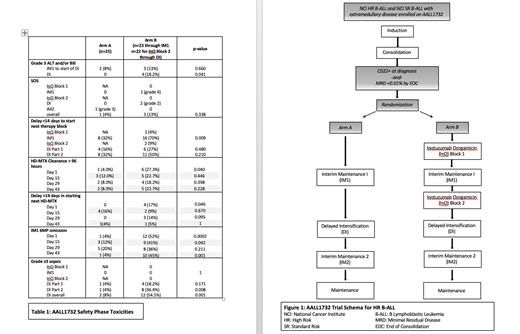
Methods: Children's Oncology Group (COG) AALL1732 is a phase 3 trial for pts 1-24 years of age with B-ALL who have high-risk features based on age, white blood cell count, or presence of extramedullary disease at diagnosis (Figure 1). Pts receive a 4-drug induction followed by augmented BFM consolidation. Those with surface CD22 expression on >20% of blasts at diagnosis and bone marrow minimal residual disease <0.01% by flow cytometry by the end of consolidation are eligible for randomization to chemotherapy alone (Arm A) or chemotherapy plus 2 cycles of InO (Arm B) delivered between consolidation and interim maintenance 1 (IM1), and between IM1 and Delayed Intensification (DI). A planned safety phase was conducted to assess hepatic toxicity, chemotherapy delays, and infectious toxicity in the first 50 randomized pts followed through the completion of DI.
Results: Forty-eight pts continued on therapy after randomization, (Arm A: 25, Arm B: 23), with median age 12 years and body surface area 1.4m 2 on each arm (Table 1). There were no statistically significant differences in grade ≥3 ALT or bilirubin levels between Arms A and B through day 1 of DI. During DI, 4 pts on Arm B had grade 3 hyperbilirubinemia compared to none on Arm A, all in the setting of concurrent infection. The rates of SOS did not cross protocol-defined safety thresholds and were not statistically different between arms; on Arm A, 1/25 pts (4%, 1 grade 3 event) and on Arm B, 3/23 pts developed SOS (13%, 1 grade 4, 2 grade 2). The patient with grade 4 SOS discontinued protocol therapy.
The duration of the IM1 block of therapy was significantly longer for patients on Arm B compared to those on Arm A (mean duration of 90 days (sd 15.3) versus 75.5 days (sd 14.4), p=0.002). Day 1 HD-MTX clearance to <0.1mM exceeded 96 hours in 6/22 (27%) pts on Arm B, compared to 1/25 (4%) on Arm A (p=0.04). There was a >14-day delay in the start of the second (Day 15) HD-MTX in 4/23 pts (17.4%) on Arm B compared to 0/25 on Arm A, p=0.046. In contrast, time to MTX clearance and rate of >96-hour clearance were not significantly different between the arms for the other HD-MTX doses (Days 15, 29, 43). Omission of oral 6MP occurred at a significantly higher frequency on Arm B compared to Arm A at several time points in IM1, with an association between HD-MTX clearance of >96 hours and the need to hold oral 6MP (p=0.011, p=0.018, and p=0.042 for days 1, 15, and 43, respectively).
During DI, sepsis occurred more frequently in pts on Arm B. There were 6 (27%) Grade 3 events and 6 (27%) ≥Grade 4 events among 22 Arm B patients, including 3 pts with multiorgan failure and one death. In contrast, among 25 pts on Arm A, there were 2 (8%) Grade 3 sepsis events and no ≥Grade 4 events. The rate of sepsis was significantly higher on Arm B (12 of 22, 54%) compared to Arm A (2 of 25, 8%, p= 0.001). The majority of sepsis events were due to bacterial infections, and 8 of 12 events on Arm B occurred in the second half of DI.
Conclusion: Planned safety phase analysis of the first 50 randomized pts demonstrated increased rates of delayed MTX clearance following the first HD-MTX infusion in IM1 and more sepsis events due to bacterial infections during DI in Arm B patients. Hepatic toxicity and SOS occurred infrequently and did not differ significantly between arms. We plan to amend AALL1732 to decrease the dose of InO by 20% and increase hydration for the first HD-MTX infusion. We will recommend prophylaxis with broad-spectrum antibiotics during periods of neutropenia in DI as well as monitoring of serum IgG levels, with replacement for IgG <400 mg/dL. A second safety phase will evaluate the effectiveness of these modifications in the next 50 randomized pts.
McNeer: Jazz Pharmaceuticals: Other: Advisory Board Member. O'Brien: Jazz: Honoraria; Pfizer: Honoraria, Research Funding. Wood: Juno, Pfizer, Amgen, Seattle Genetics: Other: Laboratory Services Agreement; Pfizer, Amgen, Seattle Genetics: Honoraria. Raetz: Pfizer: Research Funding; Celgene: Other: DSMB member. Loh: MediSix therapeutics: Membership on an entity's Board of Directors or advisory committees.